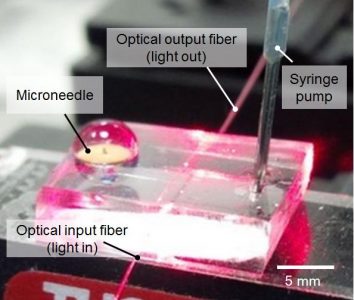
Scientists at UBC and the Paul Scherrer Institut (PSI) in Switzerland have created a microneedle drug monitoring system that could one day replace costly, invasive blood draws and improve patient comfort. These microneedles are being commercialized by Microdermics Inc., a UBC spin-off company born from the generative collaboration between Prof. Hafeli from the Faculty of Pharmaceutical Science and Prof. Stoeber from the Faculty of Applied Sciences.
Using this new system, blood-levels of a drug can be monitored by pressing a thin patch of microneedles against a patient’s arm during medical treatment. The tiny projections, less than half a milimetre long, don’t pierce the skin like a standard hypodermic needle. Instead, these microneedles extract extremely small amounts of interstitial fluid to measure drug concentrations. “This is probably one of the smallest probe volumes ever recorded for a medically relevant analysis,” said Prof. Hafeli. In fact, the volumes needed for analysis using the new system are over three orders of magnitude smaller than what is commonly used now.
Working with this tiny volume, the research team developed a way of analyzing drug levels from within the microneedle. The system includes a micro-reactor inside the hollow microneedles that traps target drugs during the procedure without having to transfer the sample and measures target concentrations with an optical biosensor.
As a first test case of the system, the research team discovered they could effectively track vancomycin levels using the microneedle drug monitoring system. The antibiotic vancomycin is used to treat serious infections. Patients taking the antibiotic undergo three to four blood draws per day and need to be closely monitored because vancomycin can cause life-threatening toxic side effects. Vancomycin is the first drug the research team has targeted with the system. They anticipate many other drugs will be effectively monitored using microneedles.
Microdermics Inc. was incorporated in 2014 and will be completing phase 1 human clinical trials for vaccine and therapeutic delivery next Spring. The company’s goal is to design and manufacture hollow metal microneedles to replace the use of hypodermic needles for many therapeutics and traditional intramuscular vaccinations.
Find out more:
Microdermics wins $110,000 BCIC First Prize Package
Scientists develop painless and inexpensive microneedle system to monitor drugs
